Significance of ipsilateral breast tumor recurrence after breast conserving treatment: role of surgical removal
Introduction
Breast conserving treatment (BCT), including primary tumor excision, axillary node dissection (determined in advance or decided following sentinel node sampling) and external beam radiation treatment (RT) to the breast, is considered standard of care for women with early-stage breast cancer in most countries. Six prospective randomized clinical trials comparing BCT to mastectomy in stage I-II invasive breast cancer did not show any significant difference between the long-term overall survivals of two treatments (1).
An important incidence of ipsilateral breast tumor recurrence (IBTR) for stage 0, I and II patients following BCT (2) has been observed after 20 years of follow-up: 8.8% following quadrantectomy plus RT (3) and 14.3% following tumorectomy plus RT (4). In particular, IBTR rates are remarkably high in patients omitting the radiation treatment: 23% at 10 years following quadrantectomy (5) and 39% at 20 years following tumorectomy (4). There are clues suggesting that IBTR may worsen the prognosis to some extent. Indeed, although no statistically significant difference in overall survival was found in any individual trial on BCT vs. mastectomy, a recent overview on local recurrences provides evidence that treatments substantially improving local control have definite, although moderate, effects on survival by 15 years (6).
A main controversial issue, lasting from the early reports, is the significance to be attributed to IBTR. The relative risk of distant metastases for patients developing IBTR in comparison with patients without IBTR is considerable, ranging from 2.11 to 4.62, depending on study features (7-9). According to some researchers, IBTR after BCT has irrelevant (7) or limited (8) detrimental effect on the natural history of the disease and it is viewed as an important marker of increased risk for, not a cause of, distant metastases (7). By contrast, others (10,11) claim that IBTR is a source of new distant metastases causing increased subsequent mortality. Recently, the controversy was sharply rekindled (12,13). The question, which is still unresolved, has theoretical implications concerning the biology of breast cancer, i.e. further support to the Fisherian “systemic” view of breast cancer compared to a more Halstedian model of the disease. Moreover, it may impinge on further treatment strategy.
The analysis of recurrence dynamics following primary tumor removal provided useful information about breast cancer metastasis development and advocated a biology-based model of breast cancer natural history that was able to explain several clinical findings (14). Therefore, we alleged that a similar analysis of the recurrence dynamics subsequent to IBTR might shed light on the biology of the subclinical tumor development also in this phase of the disease. To this purpose, we scrutinized a database including patients enrolled in a series of randomized clinical trials with similar eligibility criteria, conducted at the Milan Cancer Institute from 1973 to 1989 and identified 338 evaluable patients experiencing IBTR. The hazard rates for clinically significant events observed during the time span following IBTR were estimated and the event dynamics was analyzed. From this study, a novel view of the question emerges, pointing out a crucial role of IBTR surgical removal.
Patients and methods
Patients
Data from patients undergoing conservative surgery within three randomized clinical trials carried out at the Milan National Cancer Institute, investigating the role of different approaches for primary tumor treatment, were scrutinized. In a first trial, patients were randomized to Halsted mastectomy or quadrantectomy, axillary dissection and radiotherapy (QuaRT). A second trial accrued patients who were randomized to QuaRT or tumorectomy plus axillary dissection and radiotherapy (TaRT). In a third trial, women were randomized to QuaRT or quadrantectomy plus axillary dissection without radiotherapy (Quad). Eligibility criteria were similar for all trials.
Treatments
Quadrantectomy involved radial breast resection with excision of 2-3 cm of normal tissue around the tumor plus the removal of a portion of overlying skin and underlying fascia whilst tumorectomy removed only the tumor mass with a margin of normal tissue of 1 cm. Since the preliminary results of the first trial on the BCT of early breast cancer (15) were reported to be as good as more aggressive resections, this treatment became routine practice in our institution and data from patients treated outside randomized clinical trials (out-trial patients) were systematically recorded. This database was scrutinized as well.
All patients given QuaRT received radiotherapy: 50 Gy (daily dose 2 Gy) with high energy plus 10 Gy (daily dose 2 Gy) as a boost with orthovoltage to the ipsilateral breast. Patients allocated to TaRT received 45 Gy (daily dose 1.8 Gy) with high energy plus 15 Gy by Iridium implant. Patients allocated to Quad did not receive any radiotherapy. All axillary node positive (N+) patients were offered systemic adjuvant treatment with Cyclophosphamide + Methotrexate + Fluorouracil (CMF) or CMF plus Doxorubicin (Dx), while no further post-surgical systemic treatment was recommended to axillary node negative (N-) patients. Adjuvant hormone therapy was not utilized within the randomized clinical trials and infrequently employed for out-trial patients, as it was not considered mandatory at that time.
Two other randomized clinical trials were utilized, which considered patients for inclusion who, following mastectomy or BCT, were found to be N+. Eligible patients with 1-3 positive axillary lymph nodes were randomly allocated to receive either 12 courses of CMF or eight courses of the same regimen followed by 4 courses of Dx, while patients with >3 positive axillary nodes were randomized to receive either Dx followed by eight courses of CMF or two courses of CMF and one course of Dx for a total of 12 courses.
Detailed description of patients, treatments and follow-up modalities of the five randomized trials, which were approved by the Ethical Committee, and of the out-trial series have been reported elsewhere (15-19). In the present investigation, 3,293 patients undergoing conservative surgery (QuaRT, TaRT and Quad) and 844 patients receiving mastectomy were analyzed.
Statistical analysis
Only first events after both the treatment of the primary tumor and the treatment of IBTR were considered. IBTR was defined as any new breast cancer localization appearing in the area of the operated breast of patients undergoing conservative surgery. Distant metastasis (DM) was defined as any breast cancer manifestation(s) in areas other than that of IBTR with the exception of the contralateral breast. Contralateral breast cancers and other non-breast primary tumors were also examined. In order to avoid the usual uncertainties related to the cause of death, deaths from any cause were studied instead of breast cancer related deaths only. In the analysis of a given event, all others were considered censoring events.
The event dynamics was analyzed in a time frame with time origin at IBTR surgical removal for events following IBTR or, otherwise, at the initial surgical treatment. The event dynamics for the considered event was studied by estimating the hazard rate with the life-table method, i.e. the conditional probability of manifesting the event in a time interval, given that the patient did not previously experience it at the beginning of the interval. A discretization of the time axis in six-month units was applied and a Kernel-like smoothing procedure (20) was adopted. In addition to the Kernel smoothing approach with discrete hazards, a flexible piecewise exponential regression model was also performed in order to obtain a smoothed estimate. Cubic B splines were used to model the baseline hazard. Boundary knots were imposed in the time origin and in the last event times. Internal knots were located at the quantiles of the event time distribution (21) when the time origin was fixed at primary tumor surgery. Approximately the same knots location was used when time origin was delayed at surgery for IBTR. The 95% confidence intervals are based on the log transformation. Statistical analysis and graphic development were carried out using software R (22). with Epi package added. Estimated discrete hazards were reported for both methods, providing joint evidence of the described patterns based on different statistical procedures.
Results
Clinical data
At a median follow-up of 130 months, 356 out of 3,293 patients undergoing breast conserving surgery displayed IBTR. A total of 11 patients with IBTR did not receive any surgical treatment for their recurring disease (8 lymphangitic diffusion, 2 refusal, and 1 concomitant severe chronic cardiac failure), while the surgical approach is unknown for 5 patients and a further 2 women were lost to follow-up early after IBTR surgical removal. Therefore, the analysis was performed on 338 patients for whom adequate information was available. The main baseline features are similar in patients of the whole series and in patients developing IBTR with the exception of age (and, of consequence, menopausal status), since the latter patients were significantly younger (Table 1).
Full table
Following the diagnosis of IBTR, the treatment was decided on an individual basis. The majority of patients [190] underwent mastectomy, while the others had further conservative surgery (tumorectomy 74, quadrantectomy 74) that was combined with RT for 43 patients (i.e. for all patients who had not previously received it and for a few others). Only a minority of patients received additional systemic therapy subsequent to local treatment (hormone therapy 52, chemotherapy 13). At a median follow-up after IBTR of 151 months, 177 patients displayed a further breast cancer recurrence (72 local recurrences and 105 distant metastases) while 41 others were diagnosed with a contralateral breast tumor or second primary (Table 2).

Full table
DM dynamics
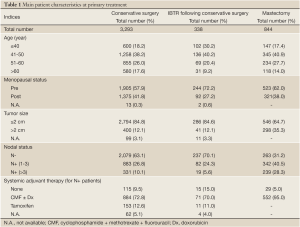
The DM dynamics following IBTR was analyzed both in a time frame with time origin at primary surgery and in a time frame with time origin at IBTR surgical removal. In the former time frame, no particular structure was observed, while in the latter one, the well-known bimodal curve appeared (Figure 1) that is typical recurrence dynamics subsequent to the treatments carried out after the diagnosis of the primary (14). The hazard rate curve displays an early peak during the second year, and a further minor peak at the sixth year.
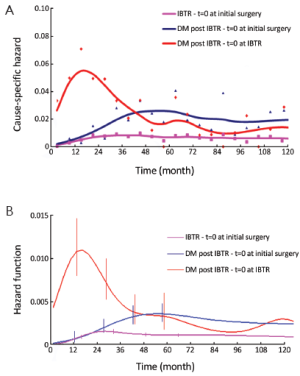
The DM dynamics observed when the time origin is set at IBTR surgical removal suggests the hypothesis that the subclinical disease course following this second surgical manoeuvre is similar to the corresponding course subsequent to the initial surgical manoeuvre. Accordingly, we investigated potential analogies between the two disease phases by comparing the recurrence dynamics for patients developing IBTR (time origin at surgery for IBTR) with the recurrence dynamics of some subsets of patients after their initial treatment (time origin at initial surgery). As reported above, following IBTR the local treatment (mastectomy or conservative surgery) was performed on an individual basis. The group of patients undergoing conservative surgery is fairly heterogeneous due to differences in surgery extent and RT administration. To rule out potential confounding effects from treatment modalities, this group should be split into smaller subsets where, however, the recurrence dynamics could not be reliably assessed. Therefore, the analysis was focused on the 190 patients receiving mastectomy at IBTR and, for comparison, on the 844 patients receiving mastectomy at initial diagnosis. The analysis of these patients revealed a noteworthy similitude between N+ patients and patients with IBTR, except for the first two years, when the risk of recurrence after IBTR is higher than after initial surgery (Figure 2, where the recurrence dynamics for N- patients is also reported).
Influence of time to IBTR
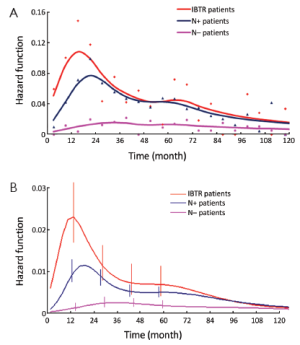
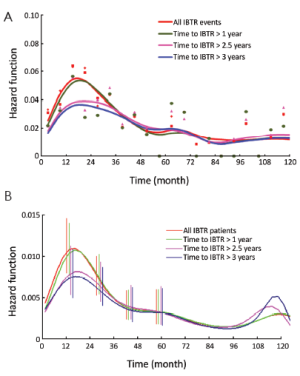
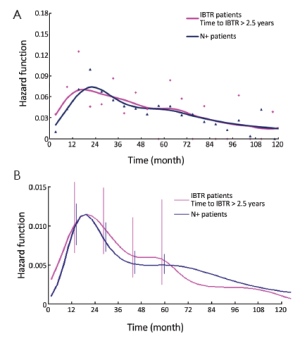
In a further step, the DM dynamics after IBTR was related to the time interval from initial surgery to IBTR. The hazard rate curves for the subsets of patients with time to IBTR exceeding 1, 1.5, 2, 2.5 or 3 years showed a first peak progressive reduction correlated to the time to IBTR, while the subsequent hazard rate pattern remained unchanged. In particular, this effect was evident for early IBTRs, while no further meaningful decrease was noticed for time to IBTR in excess of 2.5 years (Figure 3). Additionally, for patients undergoing mastectomy for IBTR when time to IBTR exceeds 2.5 years, the further recurrence dynamics was nearly superimposable to the corresponding dynamics displayed by N+ patients after primary tumor removal (Figure 4).
Mortality
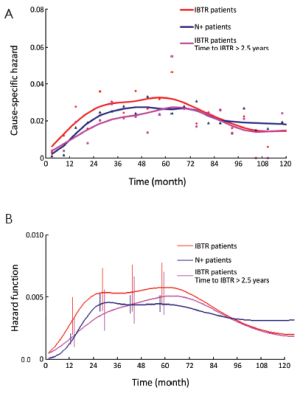
To further investigate the analogy between the clinical course of IBTR patients and N+ patients, we analyzed the mortality dynamics, regardless of the surgical approach. The resulting hazard rate curves (Figure 5) display a very similar pattern with a modestly increased level for IBTR patients during the first 6-7 years. However, when the analysis was performed for patients with time to IBTR exceeding 2.5 years, the resulting hazard rate curve was virtually identical to the corresponding curve of N+ patients.
Discussion
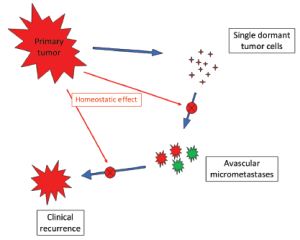
Patients with early breast cancer undergoing primary tumor removal (plus adjuvant chemotherapy for N+ cases) display a recurrence dynamics including an early major peak at the 2nd year after surgery, a smaller late peak near the 6th year and then a tapered plateau-like tail extending at least up to 15 years (14). This event dynamics has been extensively validated in 12 independent databases and also is identifiable in at least 8 other studies (23). The peak timing proved to be stable for all analyzed subsets (grouped by tumor size, nodal status, oestrogen receptor content, and menopausal status) of patients undergoing both radical and conservative surgery (14,24-26), and for all sites of metastasis (26). Such stability allows us to avoid detailed scrutiny of some characteristics (e.g., age, tumor size, whether they had adjuvant therapy, etc.) of analyzed patients who, moreover, had comparable treatments (surgery followed by similar adjuvant chemotherapy for N+ tumor and no further treatment for N- tumor). This recurrence dynamics may be suitably explained by a new paradigm of metastasis development based on the concepts of tumor homeostasis, tumor dormancy and surgery related enhancement of metastasis growth (14). The model (Figure 6) assumes both cellular and micrometastatic tumor dormancy, with ordered transitions between these two quiescent states and subsequent development of overt metastasis and, in addition, a transient phase of acceleration of metastatic growth following surgical excision of the primary tumor (14,27).
In this study, the bimodal hazard rate pattern for DM after IBTR, which emerges when times are realigned as if IBTR was a newly diagnosed breast cancer (Figure 1), suggests that the disease course following IBTR may be similar to the course subsequent to the first surgical manoeuvre. We attribute this noteworthy similarity to the IBTR surgical removal that, like primary tumor removal, impinges on the tumor-host equilibrium. We propose, therefore, that the surgery effect on tumor growth, well recognized in animals and human (27) and which reasonably explains several clinical events after primary treatment (14,23), is also operating during the treatment of IBTR.
Let us assume that IBTR removal originates an accelerating effect upon dormant micrometastases. After primary surgery, the tumor cell burden prone to wake up is time dependent, due to the orderly transitions between cellular and micrometastatic dormant states (14) (Figure 6). There is a progressive shift towards the avascular micrometastatic dormant phase that will cease following the emergence of an angiogenic phenotype (14,24). The IBTR surgical removal is a new perturbing factor of this on-going process, initiating a sudden growing phase for tumor foci most of which, otherwise, would have reached the clinical level according to their own dynamics. The recruited subclinical metastases, therefore, will emerge earlier as overt metastases. This phenomenon should be mostly relevant during early follow-up after initial surgery (the metastasis development phase underlying the first dominant risk peak), thus explaining the exceedingly high early risk for DM after IBTR in comparison with the corresponding risk for N+ patients after primary tumor removal (Figure 2). This explanation is also well supported by the reduction of the peak when patients with increasing time to IBTR are considered (Figure 3). Moreover, the assumption provides a biological reason for the often reported cut-off value of 2-3 years separating IBTR patients with worse prognosis from patients with better outcome (2).
The recurrence risk estimates suggest that patients with time to IBTR in excess of 2.5 years are similar to N+ patients (Figure 4). This result is confirmed by the mortality dynamics where an early mortality risk excess for patients with IBTR in comparison with N+ patients disappears when only patients for whom IBTR occurred at more than 2.5 years of follow-up are considered (Figure 5). It should be noted, however, that the risks of recurrence and mortality for N+ patients and IBTR patients are estimated at different times, as the time origin is at primary surgery for the former group and at IBTR surgical removal for the latter group. The recurrence and mortality risk levels associated to IBTR emerge when the corresponding risks for N+ patients may have reached low levels, because of the elapsed time from the initial surgery. Therefore, the clinical prognosis of a patient with IBTR at more than 2.5 years of follow-up may rank considerably poorer than that of an N+ patient with similar recurrence-free follow-up.
Since early reports, it was speculated that an IBTR following BCT may represent either a true recurrence of the original tumor, or, otherwise, a new primary tumor (8,28,29). A variety of empiric criteria were proposed to distinguish between the two possibilities, yet without reaching a commonly accepted standard. In our analysis, we did not distinguish between the two events and our conclusions leave out of consideration such a distinction, the validity of which needs further investigations. With this caveat, our analysis supports the concept that when an IBTR is diagnosed and resected, the patient discloses a previously unrecognized high risk of recurrence and a favorable occasion for systemic treatment as well. Therefore, we advocate investigations on the value of an adjuvant therapy following IBTR removal, such as the international multicentre trial by the International Breast Cancer Study Group and the National Surgical Adjuvant Breast and Bowel Project that has been recently initiated to address this issue.
In summary, we propose that the surgical treatment due to IBTR diagnosis induces a change in the previous DM dynamics with a sudden acceleration of metastasis development. The resulting disease course will consequently be related to both the underlying time-dependent subclinical metastatic status and the growth enhancing effect of the IBTR treatment. The finding of an overlarge early recurrence risk of IBTR patients in comparison with N+ patients, while patients with time to IBTR exceeding 2.5 years behave as N+ patients supports this notion. In addition, these findings strengthen the Fisherian concept (7) that patients with IBTR have an intrinsic high risk of DM that was undetectable by the usual prognostic factors at the initial treatment and that is revealed by the IBTR that emerges, for them, in advance of the competing DM event. Had the patient not displayed IBTR, the DM would have occurred in the majority of such patients, according to the bimodal dynamics driven by the initial treatment. Moreover, it is possible that although the majority of IBTR patients would have developed a DM in any case, in a few other patients, IBTR surgical removal may induce a growing phase of tumor foci that would have otherwise remained dormant for a long time. We suggest that these are the patients that produce the modest late mortality increase observed in the meta-analysis (6). In the choice of the surgical approach for patients with IBTR, the need of further surgery for a second IBTR following BCT for the first one should be taken into account.
The clinical course of patients experiencing IBTR may be reasonably explained by assuming jointly both the B. Fisher’s paradigm (13) and the concepts underlying the metastasis development model proposed by us (14), without the need of other explanations (12).
Acknowledgements
We wish to thank Dr. Patrizia Boracchi (Medical Statistics and Biometry, University of Milan, 20133 Milan, Italy) for useful discussions, insightful comments and critical reading of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol 2005;28:289-94.
- Huston TL, Simmons RM. Locally recurrent breast cancer after conservation therapy. Am J Surg 2005;189:229-35.
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy foe early breast cancer. N Engl J Med 2002;347:1227-32.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New Engl J Med 2002;347:1233-41.
- Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol 2001;12:997-1003.
- Early Breast Cancer Trialist’s Collaborative Group (EBCTCG), Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16.
- Fisher B, Anderson S, Fisher ER, et al. Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet 1991;338:327-31.
- Veronesi U, Marubini E, Del Vecchio M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst 1995;87:19-27.
- Whelan T, Clark R, Roberts R, et al. Ipsilateral breast tumor recurrence post-lumpectomy is predictive of subsequent mortality: results from a randomized trial. Int J Radiat Oncol Biol Phys 1994;30:11-6.
- Arriagada R, Rutqvist LE, Mattsson A, et al. Adequate locoregional treatment for early breast cancer may prevent secondary dissemination. J Clin Oncol 1995;13:2869-78.
- Fortin A, Larochelle M, Laverdière J, et al. Local failure is responsible for the decrease in survival for patients with breast cancer treated with conservative surgery and postoperative radiotherapy. J Clin Oncol 1999;17:101-9.
- Rabinovitch R, Kavanagh B. Double Helix of breast cancer therapy: intertwining the Halsted and Fisher hypotheses. J Clin Oncol 2009;27:2422-3.
- Fisher B, Anderson SJ. The breast cancer alternative hypothesis: is there evidence to justify replacing it? J Clin Oncol 2010;28:366-74.
- Demicheli R, Retsky MW, Hrushesky WJ, et al. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat Clin Pract Oncol 2007;4:699-710.
- Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med 1981;305:6-11.
- Veronesi U, Volterrani F, Luini A, et al. Quadrantectomy versus lumpectomy for small size breast cancer. Eur J Cancer 1990;26:671-3.
- Veronesi U, Luini A, Del Vecchio M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med 1993;328:1587-91.
- Moliterni A, Bonadonna G, Valagussa P, et al. Cyclophosphamide, methotrexate, and fluorouracil with and without doxorubicin in the adjuvant treatment of resectable breast cancer with one to three positive axillary nodes. J Clin Oncol 1991;9:1124-30.
- Buzzoni R, Bonadonna G, Valagussa P, et al. Adjuvant chemotherapy with doxorubicin plus cyclophosphamide, methotrexate, and fluorouracil in the treatment of resectable breast cancer with more than three positive axillary nodes. J Clin Oncol 1991;9:2134-40.
- Ramlau-Hansen H. Smoothing counting process intensities by means of Kernel functions. Ann Statist 1983;11:453-66.
- Rosenberg PS. Hazard function estimation using B splines. Biometrics 1995;51:874-87.
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R-Foundation for Statistical Computing. 2009.
- Retsky MV, Demicheli R, Hrushesky WJ, et al. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS 2008;116:730-41.
- Demicheli R, Miceli R, Brambilla C, et al. Comparative analysis of breast cancer recurrence risk for patients receiving or not receiving adjuvant cyclophosphamide, methotrexate, fluorouracil (CMF). Data supporting the occurrence of ‘cures’. Breast Cancer Res Treat 1999;53:209-15.
- Demicheli R, Biganzoli E, Ardoino I, et al. Recurrence and mortality dynamics for breast cancer patients undergoing mastectomy according to estrogen receptor status: different mortality but similar recurrence. Cancer Sci 2010;101:826-30.
- Demicheli R, Biganzoli E, Boracchi P, et al. Recurrence dynamics does not depend on the recurrence site. Breast Cancer Res 2008;10:R83.
- Demicheli R, Retsky MW, Hrushesky WJ, et al. The effects of surgery on tumor growth: a century of investigations. Ann Oncol 2008;19:1821-8.
- Recht A, Silen W, Schnitt SJ, et al. Time-course of local recurrence following conservative surgery and radiotherapy for early stage breast cancer. Int J Radiat Oncol Biol Phys 1988;15:255-61.
- Kurtz JM, Spitalier JM, Amalric R, et al. The prognostic significance of late local recurrence after breast-conserving therapy. Int J Radiat Oncol Biol Phys 1990;18:87-93.


