Primary small cell carcinoma of kidney after renal transplantation: a case report and literature review
Introduction
Small cell carcinomas (SCCs) occur most commonly in the lung. Extrapulmonary small cell carcinoma (EPSCC) is a rare neoplasm comprising 2.5% to 5% of SCCs (1). Although the EPSCC can present in various organs, including the esophagus, stomach, pancreas, gallbladder, uterine cervix, urinary bladder, kidney and prostate (2), the most common site of EPSCC is the genitourinary tract. SCCs of the genitourinary tract usually occur in the bladder. Primary renal SCC, which may occur in either the renal pelvis or the parenchyma, is extremely rare (2). Therefore, knowledge of its clinical course, presenting symptoms, appropriate treatment modality, outcomes and survival predictors is limited. We present a case of primary SCC of the kidney and review the related literatures.
Case report
In July, 2011, a 59-year-old female, who had received renal transplantation 10 years earlier, presented with left flank soreness for several weeks. Since her transplantation surgery, she had regularly taken immunosuppressive agents, including tacrolimus, mycophenolate mofetil and prednisolone. She denied other associated symptoms or signs such as gross hematuria, left flank palpable mass and abdominal pain. She denied any history of smoking. Routine laboratory examinations, including renal function tests, were unremarkable. However, ultrasonography showed a left heterogenous renal tumor and abdominal computed tomography (CT) revealed a large, complex heterogenous mass with central necrosis occupying the left kidney with no associated retroperitoneal lymphadenopathy. Therefore, renal cell carcinoma was initially suspected (Figure 1). Subsequent retrograde pyelography revealed a filling defect over the left ureteropelvic junction.
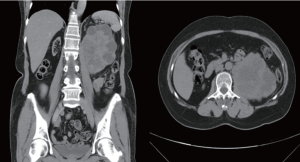
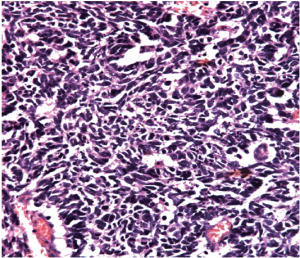
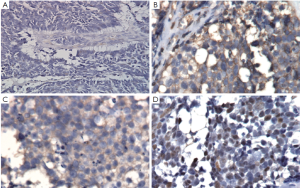
We then performed left nephroureterectomy with bladder cuff resection. The subsequent pathology results showed SCC under light microscopic examination and immunohistochemical staining. Light microscopic examination also showed that the tumor was composed of small cells, round to fusiform in shape with scanty cytoplasm, fine granular nuclear chromatin, absence of nucleoli and high mitotic activity (Figure 2). Immunohistochemical staining were positive for chromograinin, synaptophysin, CD56 and thyroid transcription factor-1 (TTF-1) (Figure 3). Metastatic workup including chest radiograph and bone scan was negative. No primary or metastatic lung lesions were noted. The pathology stage was T4N0M0 [American Joint Committee on Cancer (AJCC) stage IV]. She then received a combined adjuvant chemotherapy regimen of carboplatin 450 mg/m2 and etoposide 100 mg/m2 every month. At her 8-month postoperative follow-up, the patient showed no recurrent or metastatic disease.
Discussion
Renal SCC occurs at various ages but usually in the sixth decade of life with slightly female predominant, including younger than the age of 40 years (3). Therefore, vigilance is warranted to enable early detection of the tumor. Unlike SCC of the lung, in which smoking is a confirmed risk factor, the specific risk factors for primary renal SCC remain uncertain. For example, a literature review by Sachin et al. found that only 23% of surveyed SCC patients had a history of smoking (4).
It is difficult to distinguish renal SCC from other kinds of cancers just from clinical presentations alone. The most common symptoms are flank pain and hematuria. However, by the time these clinical symptoms occur, the tumor is usually already large with evidence of extensive spread or distant metastasis (5). Because of the aggressive clinical course of renal SCC with early dissemination and frequent recurrence, prognosis is usually poor. Based on these observations, some researchers have concluded that SCC may be associated with a high incidence of occult metastases and even localized disease (5).
Despite the wide spectrum of methods for diagnosing SCC, few provide adequate specificity. Final results depend on pathology and immunohistochemical examination. Clinically, image studies, such as sonography, abdominal CT and magnetic resonance image (MRI), have important roles in detection when early-stage SCC is suspected. Since the tumors are usually composed of necrotic areas, they show predominant hyperechoicity with anechoic areas on sonography, hypovascularity with avascular regions on angiography and heterogenous enhanced masses with hypodense areas on abdominal CT (6). Under MRI, renal SCC typically demonstrates diminished signals on T1-weighted images and heterogenous mixed signals on T2-weighted images (7). Because primary renal SCC is rare, we must survey the possibility of metastases from lung which is the most common primary SCC site including chest X-ray or CT. Light microscopic examination shows that the tumor is composed of small cells, round to fusiform in shape with scanty cytoplasm, fine granular nuclear chromatin and absent or inconspicuous nucleoli. Mitotic activity is also high (8). Immunohistochemical study is an important diagnostic tool, since one or more general neuroendocrine markers such as synaptophysin, neuron-specific enolase (NSE) and chromogranin A are positive in SCC (9).
Like SCC of the lung, renal SCC is staged as either limited or extensive. Another useful staging system is the TNM system used in the present study. Notably, the staging is not associated with survival rate and prognosis. Two theories of the histopathogenesis of urinary tract SCC have been proposed. One theory indicates that it may arise from the neuroendocrine cells in the urinary tract because of immunohistochemical neuroendocrine feature finding. The other is that it transforms from pluripotent epithelial reserve cells capable of differentiating into various cell types. Clinically, SCC of the renal pelvis may coexist with urothelial carcinoma, adenocarcinoma or squamous cell carcinoma (3). This phenomenon supports the latter theory (2).
Renal SCC may be located in parenchyma or renal pelvis which presents some differences between each other. A SCC emerging in the parenchyma is purely a neuroendocrine carcinoma while a SCC emerging in the renal pelvis occurs in combination with non-neuroendocrine carcinoma such as urothelial carcinoma, squamous cell carcinoma or glandular carcinoma (9). In the reported case, although it is difficult to distinguish from parenchyma or renal pelvis origin grossly due to huge tumor, the pathologist did not discover concomitant non-neuroendocrine carcinoma. Based on the above theory, the renal SCC in the reported case may have originated from parenchyma.
Due to the rarity of renal SCC and its aggressive characteristics, definitive treatment protocols have not been established. However, since surgery alone does not improve survival rate, many clinicians suggest a multimodality therapy including surgery, chemotherapy and radiotherapy. For SCC of lung, a combination of a platinum-based chemotherapeutic agent and etoposide is one of the most used regimens. Platinum-based chemotherapy reportedly achieves a higher survival rate compared to other forms of chemotherapy (4). The effectiveness of radiotherapy has not been clearly established because of the widely varying results reported in the literature. The most common metastatic or recurrent sites are bone, liver, lung and brain. Therefore, careful survey of the abdomen, chest and even the brain must be considered in the follow-up assessments. For early diagnosis of metastases, 6 months of close monitoring of patients is recommended (6). According to Sachin et al., immunoreactivity with vimentin and carcinoembryonic antigen (CEA) indicates poor prognosis and development of early metastases with potentially poor survival rate (10,11). Although the precise mechanism is unclear, we hypothesize that the patient reported here developed an increased risk of carcinoma formation after taking immunosuppressant for several years.
In conclusion, because primary renal SCC presents with an advanced tumor stage and a short median survival period, early intervention and close follow-up are recommended. In addition to surgical nephrectomy, platinum-based chemotherapy is associated with prolonged survival (5).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Walenkamp AM, Sonke GS, Sleijfer DT. Clinical and therapeutic aspects of extrapulmonary small cell carcinoma. Cancer Treat Rev 2009;35:228-36. [PubMed]
- Kho VK, Chan PH. Primary small cell carcinoma of the upper urinary tract. J Chin Med Assoc 2010;73:173-6. [PubMed]
- Si Q, Dancer J, Stanton ML, et al. Small cell carcinoma of the kidney: a clinicopathologic study of 14 cases. Hum Pathol 2011;42:1792-8. [PubMed]
- Patil S, Kaza RC, Kakkar AK, et al. Small cell carcinoma of the renal pelvis: a case report and review of the literature. ISRN Urol 2011;2011:786505.
- Majhail NS, Elson P, Bukowski RM. Therapy and outcome of small cell carcinoma of the kidney: report of two cases and a systematic review of the literature. Cancer 2003;97:1436-41. [PubMed]
- Lee SY, Hsu HH, Lin HY, et al. Factors associated with the survival of patients with primary small cell carcinoma of the kidney. Int J Clin Oncol 2013;18:139-47. [PubMed]
- Karadeniz-Bilgili MY, Semelka RC, Hyslop WB, et al. MRI findings of primary small-cell carcinoma of kidney. Magn Reson Imaging 2005;23:515-7. [PubMed]
- Martin SM, Gonzalez JR, Lagarto EG, et al. Primary small cell carcinoma of the ureter. Int J Urol 2007;14:771-3. [PubMed]
- La Rosa S, Bernasconi B, Micello D, et al. Primary small cell neuroendocrine carcinoma of the kidney: morphological, immunohistochemical, ultrastructural, and cytogenetic study of a case and review of the literature. Endocr Pathol 2009;20:24-34. [PubMed]
- Chuang CK, Liao SK. A retrospective immunohistochemical and clinico- pathological study of small cell carcinomas of the urinary tract. Chang Gung Med J 2003;26:26-33. [PubMed]
- Goslin RH, Skarin AT, Zamcheck N. Carcinoembryonic antigen. A useful monitor of therapy of small cell lung cancer. JAMA 1981;246:2173-6. [PubMed]


