Prognostic value of post-treatment 18F-FDG PET/CT for advanced head and neck cancer after combined intra-arterial chemotherapy and radiotherapy
Introduction
For early metastasis or recurrence detection in patients with head and neck squamous cell carcinoma (HNSCC), structural imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) have been developed (1). However, radiation and/or surgery can distort subsequent imaging findings and diagnosis using conventional imaging at an early stage of recurrences or residual tumors may be difficult (2). Combined intra-arterial chemotherapy and radiotherapy (IACR) has become an important organ-sparing approach for treating HNSCC (3,4). In overall survival (OS), superselective IACR using high-dose cisplatin (CDDP) has outcomes similar to those of intravenous chemoradiotherapy, although the site of toxicity varies (5,6). However, a drawback of this treatment is the high incidence of residual lesions, which makes it difficult to distinguish recurrences from scars.
The advent of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) has improved the treatment evaluation in patients with HNSCC after IACR (7-10). Additionally, several studies have reported the usefulness of pretreatment 18F-FDG PET/CT evaluations for diagnostic and prognostic values (11-15). However, few studies have reported the prognostic value of post-treatment 18F-FDG PET/CT in patients with HNSCC undergoing IACR.
This study aimed to clarify the prognostic value of post-IACR 18F-FDGPET/CT for disease outcome and survival in patients with HNSCC.
Materials and methods
Subject characteristics
Thirty-six consecutive patients with advanced HNSCC who underwent initial definitive therapy from April 2008 to March 2011 were recruited for this study. All patients who received initial treatment had advanced cancer [TNM classification of malignant tumor (TNM) cancer staging 3 or 4], assessed on the basis of the American Joint Committee on Cancer staging. None of the patients received pre-treatment 18F-FDG PET/CT at our hospital for various reasons such as 18F-FDG PET/CT was performed at their former clinics, full reservation of 18F-FDG PET/CT departments or sudden change of schedule in operating departments, errors in the 18F-FDG PET/CT reservation by patients or physicians, or physicians with negative attitudes toward pretreatment 18F-FDG PET/CT. No patient had any signs of distant metastasis or second primary malignancy during IACR, which was revealed by both conventional imaging (including enhanced CT scanning from the neck to the upper abdomen) and physical examinations. After the histological findings were confirmed, all patients were treated using IACR. Oral or written informed consent was obtained from all patients. This study was approved by the institutional review board of our hospital.
The weekly chemotherapy regimen involved the following steps: superselective intra-arterial infusion of 150 mg/m2 CDDP was administered (3 or 4 times) with concurrent radiotherapy over seven weeks starting concurrently with chemotherapy. Dose modification was made depending on patient age; for patients over 75 years of age, 50-70% of the full doses were administered. Systemic antagonization of CDDP with sodium thiosulfate was used during intra-arterial infusion. A standard external radiotherapy regimen using 60-72 Gy/30-36 fractions in 6-8 weeks at 2 Gy/d 5 days a week was administered. The dose administered to the spinal cord was limited to 40 Gy, and re-evaluation was performed by physical examination, CT, and/or MRI to monitor reduction in tumor size. Finally, when reduction was confirmed, an additional dose was administered to the reduced field, excluding the spinal cord.
A coaxial microcatheter system and the standard Seldinger technique were used to perform superselective catheterization through the femoral, brachial, or superficial temporal artery under local anesthesia. For the coaxial system, a 2.3-Fr microcatheter (Prowler SELECT PLUS; Johnson & Johnson, New Brunswick, New Jersey, USA) and guidewire were introduced through a 5-Fr diagnostic catheter (ENVOY; Codman & Shurtleff, Inc., Raynham, Massachusetts, USA). The catheter tip was placed in the external carotid artery or common carotid artery under systemic heparinization to prevent thromboembolic complications related to the catheter procedure. The microcatheter tip was advanced as distally as possible, and an appropriate feeding artery was identified. Interventional-radiology computed tomography (IVR-CT) and digital subtraction angiography (INFX-8000C; Toshiba Medical Systems Co., Otawara, Japan) were performed. The iodine contrast media flow rate was 1 mL/s, as observed on IVR-CT. Chemotherapeutic agents were administered through the feeding arteries, with the dose depending on the volume of vascular territory.
Follow-up procedures
After treatment completion, each patient was followed up until dropout or patient death during the observation period. As part of the surveillance protocol for detection of recurrences or residual tumors, all subjects underwent 18F-FDG PET/CT. The period from the end of IACR to the last post-treatment 18F-FDG PET/CT examination was 8-12 weeks. No patient had any signs of infection (e.g., high fever, severe elevation in white blood cell count, serum C-reactive protein, focal pain) during the post-treatment 18F-FDG PET/CT scan. During the treatment and follow-up periods, board-certified dentists checked oral side effects, including mucosal damage. All patients were allowed oral intake by 8 to 12 weeks after IACR.
To detect locoregional recurrences in the neck region, post-treatment assessments using flexible endoscopy and/or indirect laryngoscopy and by physical examination as well as 18F-FDG PET/CT examination were performed for each patient within at least three months after IACR. An enhanced CT and/or MRI follow-up examination was performed at the discretion of an experienced clinician. The governing criteria to define recurrences were re-growth of lesions at the primary tumor sites, appearance of lesions at the distant organs, or appearance or re-growth of an enlarged lymph node with a size (approximately >15 mm in maximum diameter) comparable to that in previous studies or observed in the CT or MRI images after completion of therapy.
Patients with 18F-FDG PET/CT-positive findings underwent aspiration or excision biopsy or clinical follow-up involving enhanced CT and/or MRI, physical examinations, and endoscopic examinations to confirm the diagnosis. All recurrences were defined as the first site of failure, including limited failure (local and/or regional failures), or distant failure. All failures were estimated on the basis of imaging or histological findings.
18F-FDG PET/CT imaging
The patients’ fasting (>4 h) blood glucose levels were measured before 18F-FDG administration. Subsequently, 4-6 MBq/kg of 18F-FDG was intravenously injected 1 h before initiating the whole-body PET/CT. The mean blood glucose level at the time of the 18F-FDG PET/CT examination was 109.0±24.5 mg/dL. All patients underwent the procedure using a combined 16-slice PET/CT scanner (Biograph 16; Siemens, Munich, Germany). After 18F-FDG injection, a PET/CT acquisition protocol was followed to scan from the vertex to the mid thighs. For this examination, CT was performed (60 mA, 120 keV, a section width of 5 mm, and 0.5 s/CT rotation) without the use of intravenous iodinated or oral contrast media. For the emission scans (3 min/bed position; 128×128 matrix) of the whole-body PET/CT protocol (at least 8 bed positions; field of view, 16.2 cm axial) in 3-dimensional mode, a standard PET/CT bed with a built-in head holder was used to scan the patients; the patient’s arms were positioned downward at their sides. The CT data, an ordered-subset expectation maximization algorithm (8 subsets and 3 iterations), and the standard manufacturer-supplied reconstruction software (e.soft; Siemens) were used to reconstruct the attenuation-corrected 18F-FDG PET/CT images.
Image interpretation
Two methods were used to evaluate recurrence: patient-based and lesion-based analyses. In the patient-based analysis, the 18F-FDG PET/CT images for each patient were categorized in a binary fashion as normal/probable normal or abnormal/probable abnormal. The following governing criteria of visual analysis were used: (I) Any foci of increased 18F-FDG uptake relative to the surrounding normal soft tissue that were not located in areas of physiologically increased uptake were considered to be positive for primary to metastatic lesions; (II) Abnormal structures on PET/CT images were considered to be positive even without significant 18F-FDG uptake (e.g., lung nodules, bone destruction). The assessments were performed by an independent experienced radiologist/nuclear medicine physician who was the chief reviewer and by four nuclear medicine physicians who reviewed the imaging results. The chief reviewer interpreted all 18F-FDG PET/CT images and checked all reports. Any differences in interpretations of the reports were resolved by consensus through contact with each nuclear medicine physician. When the physicians surveyed the images and reports, they were aware of the patients’ histories and the clinical findings or previous imaging results obtained before IACR. After visually examining all of the images on a computer display and workstation (e.soft V; Siemens), the reviewers finalized the diagnoses mainly on the basis of the PET and CT fusion images to accurately confirm the physiological accumulation and to exclude active uptake resulting from radiotherapy or surgery. The reviewers had access to all PET and CT images, including multiplanar reconstructions.
In the lesion-based analysis, regions of interest (ROIs) determined on the basis of the visual assessment were placed in 36 regions of 36 patients (1 region selected in each patient), which included 12 recurrences (6 limited lesions and 6 distant metastases) showing maximum 18F-FDG uptake in 12 patients with recurrence within six months after the end of treatment, and 24 scars at the primary tumor sites in 24 patients without recurrence within six months after the end of treatment. The same workstation was used to delineate ROIs in each region to obtain the maximum standardized uptake value (SUVmax) on transaxial images.
Statistical analysis
The statistical endpoints analyzed in this study were local control, regional control, distant control, and OS measured from IACR to the event date; the patients’ data were censored at the last follow-up or death. The Kaplan-Meier method was used to estimate the probabilities of tumor control and survival rates at two years, irrespective of follow-up length. The two-tailed log-rank test was performed to compare the survival rates among the groups. Standard formulae were used to calculate the sensitivity, specificity, and accuracy. Unpaired t-tests and Pearson χ2 tests (or Fisher’s exact tests) were used to examine differences (i.e., in SUVmax) between the groups (recurrence-positive vs. recurrence-negative).
Conventional receiver-operating characteristics (ROC) curve analysis was used to examine SUVmax. The level of statistical significance was set to P<0.05. PASW version 18.0 for Windows (IBM, New York, USA) was used for all analyses.
Results
Patient characteristics and validation of recurrence
The primary tumor sites and TNM staging are summarized in Table 1. The mean follow-up period was 23.8 (range, 4-47) months. Ultimately, recurrence was observed in 15 patients, and 9 died during the observation period. The reference standard analyses (histology or follow-up) revealed tumor recurrences in 15 (42%) patients during the overall observation period. Of these, there were limited recurrences after treatment in 7 sites and distant metastases in 8. Six months after IACR, there were recurrences in 6 of the 7 limited sites and 6 of the 8 distant metastases.

Full table
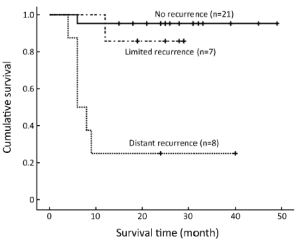
The disease-free survival results are shown in Figure 1. Survival analysis using the Kaplan-Meier method revealed that there were significant differences in OS among the three groups. The estimated mean OS was significantly lower for the distant recurrence group [14.9 months; 95% confidence interval (95% CI), 4.8-25.0 months] than for the limited recurrence (26.6 months; 95% CI, 22.2-31.0 months) and no recurrence groups (47.0 months; 95% CI, 43.0-50.9 months) (P<0.05 and P<0.001, respectively).
Outcomes of patient-based and lesion-based analyses
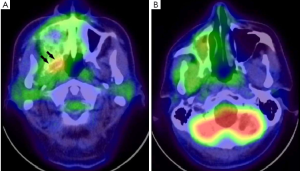
The patient-based analysis revealed that the 18F-FDG PET/CT findings were negative in 25 patients and positive in 11 (Figure 2). The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 18F-FDG PET/CT for diagnosing recurrence within six months after treatment were 67% (8/12), 88% (21/24), 73% (8/11), 84% (21/25), and 81% (29/36), respectively.
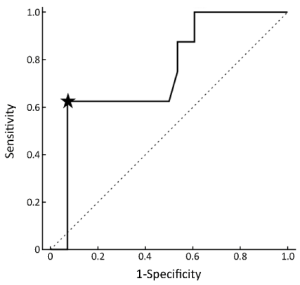
The lesion-based analysis revealed that SUVmax of 18F-FDG was 4.46±2.73. SUVmax was 7.02±3.17 for recurrence (n=12) and 3.19±1.19 for non-recurrence (n=24) (P<0.001). The results from an analysis of SUVmax six months after IACR (Figure 3) were used to plot the conventional ROC curves. The ROC curve analysis showed that a representative SUVmax cut-off point of 6.1 had a maximal sensitivity of 63% and a specificity of 93%.
Prognostic value of 18F-FDG PET/CT
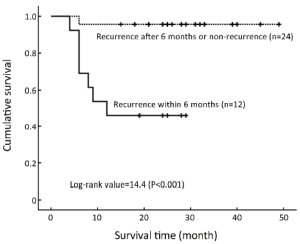
Twelve of the 15 patients with recurrence and 8 of the 9 who had died were identified by six months and two years after IACR, respectively. OS was lower in the group with recurrence within six months than in the group with recurrence after six months (P<0.01; Figure 4).
We evaluated two factors associated with 18F-FDG PET/CT (SUVmax and visual interpretation) to stratify OS in the two groups with and without recurrence. SUVmax of 6.1 was selected as the cut-off point from the ROC curve. The Kaplan-Meier analysis indicated that OS was significantly higher in the lower SUVmax group than in the higher SUVmax group (P<0.001; Figure 5A). The mean OS was estimated to be 12.1 months (95% CI, 6.3-18.0 months) for the higher SUVmax group (n=7) and 44.6 months (95% CI, 39.9-49.3 months) for the lower SUVmax group (n=29). In addition, OS was significantly higher in the negative visual interpretation group than in the positive SUVmax group (P<0.05; Figure 5B).

Discussion
Our findings suggested that SUVmax and visual interpretation of post-IACR 18F-FDG PET/CT images can be prognostic indicators of outcome for at least two years, although the longer-term predictive value may be less clear. The differentiating feature of this study was that patients with HNSCC who underwent IACR were investigated. In addition, this result suggests that post-treatment 18F-FDG PET/CT may be useful even for patients who have not undergone pre-treatment 18F-FDG PET/CT.
Several studies have reported the prognostic value of 18F-FDG PET/CT uptake in HNSCC before treatment (11-17). In general, these studies revealed that patients with higher SUVs (mean or max) in primary lesions, higher total metabolic tumor volumes, or higher total lesion glycolysis were associated with poor prognosis or disease-free survival (18,19). However, these prognostic values strongly depend on success of the treatment. On the other hand, few studies have reported the prognostic value of post-treatment 18F-FDG PET/CT, even though a meta-analysis reported usefulness of post-treatment 18F-FDG PET (14,20). Generally, 18F-FDG uptakes in the post-treatment state within a few weeks after chemoradiotherapy were modified because of inflammatory changes caused by radiation therapy (21). After chemoradiotherapy, glucose metabolism increases in lymph nodes, salivary glands, muscles, and soft tissue. Accumulation of 18F-FDG due to these inflammatory factors, which affects differential diagnosis, decreases over time (22). These inflammatory changes have hampered correct interpretation of post-treatment 18F-FDG PET/CT. However, hasty evaluation for therapeutic effect and earlier detection for recurrence are critical for patients with malignancy. Recently, several studies reported that accurate evaluation was possible with 18F-FDG PET/CT performed 8-12 weeks after the end of chemoradiotherapy (8,21,23,24). In addition to their results, our results support the possibility that post-treatment 18F-FDG PET/CT may be useful for predicting prognosis even in earlier follow-ups after the end of treatment.
A previous study reported that post-treatment 18F-FDG PET/CT has diagnostic value for identifying recurrences within six months after IACR (8). On the basis of this result, we consider that the prognostic and diagnostic value of 18F-FDG PET/CT may be limited to within a certain time period. Furthermore, we selected two years as an appropriate observation period for OS because other studies had shown that HNSCC patients with positive 18F-FDG uptake had poor prognosis within two years (14). Our results suggested that 18F-FDG PET/CT findings predicts prognosis at least within two years.
The limitations of this retrospective study include the mixed population and the heterogeneity of the regions investigated. In addition, most of the patients did not undergo pre-treatment 18F-FDG PET/CT. Therefore, the study population may have a selection bias with regard to the number of patients with HNSCC. However, this study has the advantage that the subjects with HNSCC completely underwent the laborious IACR protocol and post-treatment 18F-FDG PET/CT. In actual clinical situations, many patients may not have a chance to undergo pre-treatment 18F-FDG PET/CT for various reasons. Our results may support that only post-treatment 18F-FDG PET/CT information is useful to determine prognosis after IACR. We believe that this study does not have many biases with respect to treatment or examination from the standpoint of realistic clinical situations. Therefore, the results of this study may be applicable to typical hospital populations.
In conclusion, our results showed that SUVmax and visual interpretation of HNSCC (with or without distant recurrence) on 18F-FDG PET/CT performed after IACR can provide prognostic survival estimates of at least two years.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Liao LJ, Lo WC, Hsu WL, et al. Detection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-a meta-analysis comparing different imaging modalities. BMC Cancer 2012;12:236. [PubMed]
- Haas I, Hauser U, Ganzer U. The dilemma of follow-up in head and neck cancer patients. Eur Arch Otorhinolaryngol 2001;258:177-83. [PubMed]
- Takayama O, Yokoyama J, Ito S. Therapeutic experience of recurrent myoepithelial carcinoma by superselective intra-arterial chemotherapy infused high-dose CDDP. Auris Nasus Larynx 2006;33:235-8. [PubMed]
- Homma A, Furuta Y, Suzuki F, et al. Rapid superselective high-dose cisplatin infusion with concomitant radiotherapy for advanced head and neck cancer. Head Neck 2005;27:65-71. [PubMed]
- Rasch CR, Hauptmann M, Schornagel J, et al. Intra-arterial versus intravenous chemoradiation for advanced head and neck cancer: Results of a randomized phase 3 trial. Cancer 2010;116:2159-65. [PubMed]
- Ackerstaff AH, Balm AJ, Rasch CR, et al. First-year quality of life assessment of an intra-arterial (RADPLAT) versus intravenous chemoradiation phase III trial. Head Neck 2009;31:77-84. [PubMed]
- Kitagawa Y, Nishizawa S, Sano K, et al. Prospective comparison of 18F-FDG PET with conventional imaging modalities (MRI, CT, and 67Ga scintigraphy) in assessment of combined intraarterial chemotherapy and radiotherapy for head and neck carcinoma. J Nucl Med 2003;44:198-206. [PubMed]
- Ito K, Yokoyama J, Kubota K, et al. 18F-FDG versus 11C-choline PET/CT for the imaging of advanced head and neck cancer after combined intra-arterial chemotherapy and radiotherapy: the time period during which PET/CT can reliably detect non-recurrence. Eur J Nucl Med Mol Imaging 2010;37:1318-27. [PubMed]
- Döbert N, Kovacs AF, Menzel C, et al. FDG uptake after intraarterial chemotherapy in head and neck cancer. Nuklearmedizin 2006;45:243-7. [PubMed]
- Döbert N, Kovacs AF, Menzel C, et al. The prognostic value of FDG PET in head and neck cancer. Correlation with histopathology. Q J Nucl Med Mol Imaging 2005;49:253-7. [PubMed]
- Lindholm P, Leskinen S, Lapela M. Carbon-11-methionine uptake in squamous cell head and neck cancer. J Nucl Med 1998;39:1393-7. [PubMed]
- Suzuki H, Kato K, Fujimoto Y, et al. (18)F-FDG-PET/CT predicts survival in hypopharyngeal squamous cell carcinoma. Ann Nucl Med 2013;27:297-302. [PubMed]
- Kim G, Kim YS, Han EJ, et al. FDG-PET/CT as prognostic factor and surveillance tool for postoperative radiation recurrence in locally advanced head and neck cancer. Radiat Oncol J 2011;29:243-51. [PubMed]
- Kao J, Vu HL, Genden EM, et al. The diagnostic and prognostic utility of positron emission tomography/computed tomography-based follow-up after radiotherapy for head and neck cancer. Cancer 2009;115:4586-94. [PubMed]
- Torizuka T, Tanizaki Y, Kanno T, et al. Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR Am J Roentgenol 2009;192:W156-60. [PubMed]
- Xie P, Li M, Zhao H, et al. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol 2011;137:1085-93. [PubMed]
- Nakajo M, Kajiya Y, Tani A, et al. FDG PET/CT and diffusion-weighted imaging of head and neck squamous cell carcinoma: comparison of prognostic significance between primary tumor standardized uptake value and apparent diffusion coefficient. Clin Nucl Med 2012;37:475-80. [PubMed]
- Schöder H, Fury M, Lee N, et al. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med 2009;50 Suppl 1:74S-88S. [PubMed]
- Kawabe J, Higashiyama S, Yoshida A, et al. The role of FDG PET-CT in the therapeutic evaluation for HNSCC patients. Jpn J Radiol 2012;30:463-70. [PubMed]
- Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol 2008;33:210-22. [PubMed]
- Quon A, Fischbein NJ, McDougall IR, et al. Clinical role of 18F-FDG PET/CT in the management of squamous cell carcinoma of the head and neck and thyroid carcinoma. J Nucl Med 2007;48 Suppl 1:58S-67S. [PubMed]
- Ryan WR, Fee WE Jr, Le QT, et al. Positron-emission tomography for surveillance of head and neck cancer. Laryngoscope 2005;115:645-50. [PubMed]
- Andrade RS, Heron DE, Degirmenci B, et al. Posttreatment assessment of response using FDG-PET/CT for patients treated with definitive radiation therapy for head and neck cancers. Int J Radiat Oncol Biol Phys 2006;65:1315-22. [PubMed]
- Ito K, Yokoyama J, Kubota K, et al. Comparison of 18F-FDG and 11C-choline PET/CT for detecting recurrences in patients with nonsquamous cell head and neck malignancies. Nucl Med Commun 2010;31:931-7. [PubMed]


